IMARC Group, a leading market research company, has recently released a report titled “Orphan Drugs Market Report by Drug Type (Biological, Non-Biological), Disease Type (Oncology, Hematology, Neurology, Cardiovascular, and Others), Phase (Phase I, Phase II, Phase III, Phase IV), Top Selling Drugs (Revlimid, Rituxan, Copaxone, Opdivo, Keytruda, Imbruvica, Avonex, Sensipar, Soliris, and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Stores, and Others), and Region 2025-2033”. The study provides a detailed analysis of the industry, including the global orphan drugs market share, trends, size, and industry growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
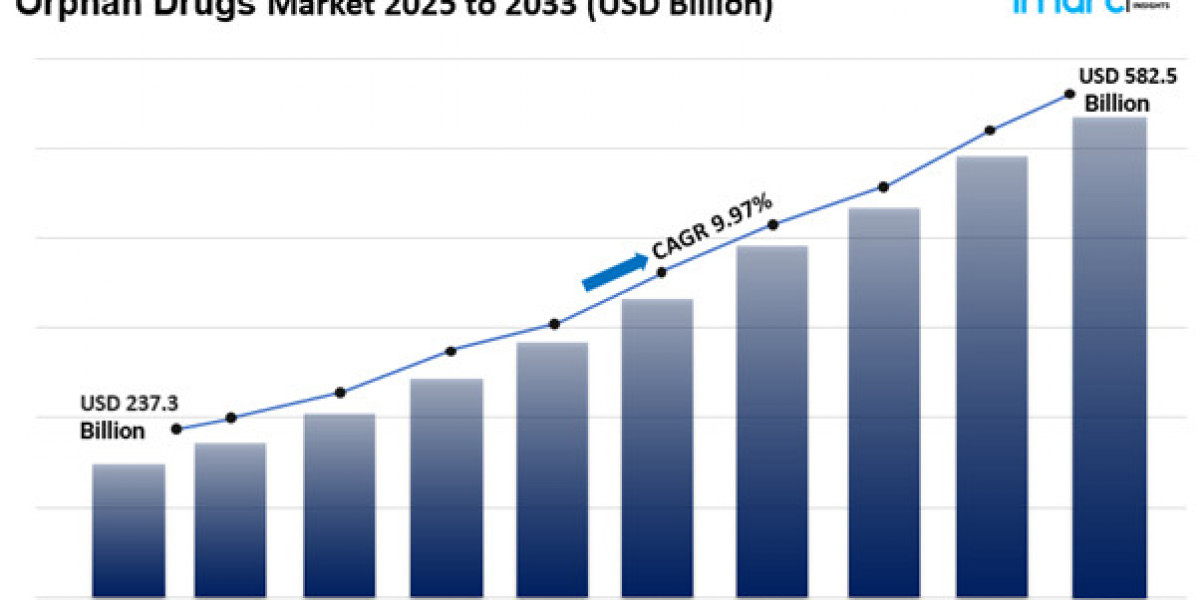
The global orphan drugs market size reached USD 237.3 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 582.5 Billion by 2033, exhibiting a growth rate (CAGR) of 9.97% during 2025-2033.
Request to Get the Sample Report:
https://www.imarcgroup.com/orphan-drugs-market/requestsample
Orphan Drugs Market Trends in 2025
The orphan drugs market is set to change as it adapts to new healthcare needs. By 2025, patient-focused approaches will become more important. Companies will prioritize the experiences of patients with rare diseases. This shift will lead to better treatment plans that include more than just medications. Support services, education, and access to healthcare will also be key.
Pharmaceutical companies will work closely with patient advocacy groups. They aim to understand the challenges that those with rare diseases face. Additionally, digital health technologies will improve patient monitoring and management. This will enhance the overall treatment experience.
Telemedicine and mobile health apps will allow better communication between patients and providers. This ensures timely support and interventions. As the orphan drugs market adopts a patient-cantered approach, it will improve treatment outcomes. It will also build trust and loyalty among patients and their families, driving market growth.
Market Dynamics of Orphan Drugs Market Trends & Demand
Growing Prevalence of Rare Diseases
The orphan drugs market is growing fast due to the rising number of rare diseases worldwide. As healthcare professionals and the public learn more about these conditions, the demand for effective treatments grows. By 2025, improved genetic research and diagnostics will help identify rare diseases more clearly. This will lead to more patients seeking treatment. Organizations and governments are increasing research funding and promoting orphan drug development.
In response, pharmaceutical companies are investing in research and development (R&D) for new orphan drugs. Partnerships between biotech firms and academic institutions will also speed up the creation of innovative therapies. As we discover more about these diseases, the orphan drugs market will expand, bringing new hope to patients and their families.
Regulatory Support and Incentives
Regulatory bodies worldwide are increasingly supporting orphan drug development. This support is changing market dynamics. By 2025, more policies will likely speed up the approval process for orphan drugs. This will help patients with rare diseases access life-saving treatments faster.
Programs like the Orphan Drug Act in the U.S. and similar rules in Europe offer financial incentives. These include tax credits and market exclusivity to encourage drug development. This regulatory support reduces financial risks for pharmaceutical companies and boosts innovation.
Moreover, patient registries and data-sharing initiatives will improve our understanding of rare diseases. This will lead to more focused research and development. As regulatory support grows, the orphan drugs market is set for strong growth. This will attract both established companies and newcomers eager to meet unmet medical needs.
Rising Investment in Biotechnology
Investing in biotechnology drives growth in the orphan drugs market. Advances in biopharmaceuticals open new paths for therapies targeting rare diseases. By 2025, biotechnology firms will be essential in discovering and commercializing orphan drugs. They will use technologies like gene therapy, monoclonal antibodies, and CRISPR gene editing. These methods create targeted treatments that tackle the root causes of rare diseases, not just symptoms.
The trend of personalized medicine is also expected to grow in this field. Treatments will be tailored to fit individual patient needs. As investors see the potential for high returns, funding for biotech startups focused on rare disease therapies will likely rise. This influx of capital will boost research and development, leading to new orphan drugs in the market. As biotechnology advances, it will greatly influence the future of the orphan drugs market.
Orphan Drugs Market Report Segmentation:
Breakup by Drug Type:
· Biological
· Non-Biological
Breakup by Disease Type:
· Oncology
· Hematology
· Neurology
· Cardiovascular
· Others
Breakup by Phase:
· Phase I
· Phase II
· Phase III
· Phase IV
Breakup by Top Selling Drugs:
· Revlimid
· Rituxan
· Copaxone
· Opdivo
· Keytruda
· Imbruvica
· Avonex
· Sensipar
· Soliris
· Others
Breakup by Distribution Channel:
· Hospital Pharmacies
· Retail Pharmacies
· Online Stores
· Others
Breakup by Region:
· North America
· Asia Pacific
· Europe
· Latin America
· Middle East and Africa
Competitive Landscape with Key Players:
The competitive landscape of orphan drugs market size has been studied in the report with the detailed profiles of the key players operating in the market.
Some of These Key Players Include:
· AbbVie Inc.
· Alexion Pharmaceuticals Inc
· Amgen Inc.
· Biogen Inc.
· Bristol-Myers Squibb Company
· F. Hoffmann-La Roche AG (Roche Holding AG)
· Jazz Pharmaceuticals Plc
· Johnson & Johnson
· Merck & Co. Inc.
· Novartis AG
· Pfizer Inc.
· Sanofi S.A.
· Takeda Pharmaceutical Company Limited
· Teva Pharmaceutical Industries Ltd.
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=2382&flag=C
Key Highlights of the Report:
· Market Performance (2018-2023)
· Market Outlook (2024-2032)
· Market Trends
· Market Drivers and Success Factors
· Impact of COVID-19
· Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Website: imarcgroup.com
Email: sales@imarcgroup.com
Americas: +1-631-791-1145 | Europe & Africa: +44-753-713-2163 |Asia: +91-120-433-0800